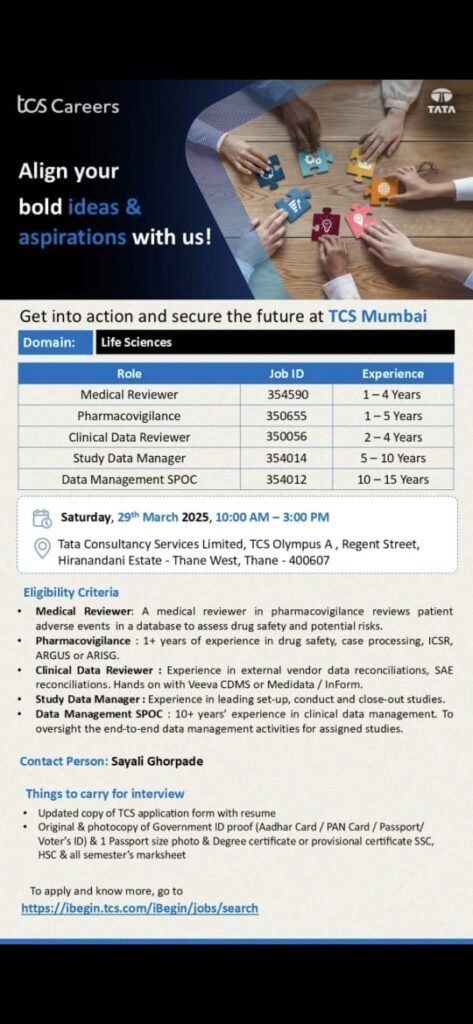
Align your bold ideas and aspirations with Tata Consultancy Services (TCS) in Mumbai! TCS is offering exciting career opportunities in the Life Sciences domain. Whether you’re a seasoned professional or just starting your career, TCS has roles that can help you secure your future.
Job Details:
- Medical Reviewer
- Job ID: 354590
- Experience: 1-4 Years
- Role: A Medical Reviewer in pharmacovigilance reviews patient adverse events in a database to assess drug safety and potential risks.
- Pharmacovigilance
- Job ID: 350655
- Experience: 1-5 Years
- Role: Requires 1+ years of experience in drug safety, case processing, ICSR, ARGUS, or ARISG.
- Clinical Data Reviewer
- Job ID: 350056
- Experience: 2-4 Years
- Role: Experience in external vendor data reconciliations, SAE reconciliations. Hands-on with Veeva CDMS or Medidata / InForm.
- Study Data Manager
- Job ID: 354014
- Experience: 5-10 Years
- Role: Experience in leading set-up, conduct, and close-out studies.
- Data Management SPOC
- Job ID: 354012
- Experience: 10-15 Years
- Role: 10+ years’ experience in clinical data management. To oversight the end-to-end data management activities for assigned studies.
Event Details:
- Date: Saturday, 29th March 2025
- Time: 10:00 AM – 3:00 PM
- Venue: Tata Consultancy Services Limited, TCS Olympus A, Regent Street, Hiranandani Estate – Thane West, Thane – 400607
Eligibility Criteria:
- Medical Reviewer: Experience in reviewing patient adverse events in a database to assess drug safety and potential risks.
- Pharmacovigilance: 1+ years of experience in drug safety, case processing, ICSR, ARGUS, or ARISG.
- Clinical Data Reviewer: Experience in external vendor data reconciliations, SAE reconciliations. Hands-on with Veeva CDMS or Medidata / InForm.
- Study Data Manager: Experience in leading set-up, conduct, and close-out studies.
- Data Management SPOC: 10+ years’ experience in clinical data management. To oversight the end-to-end data management activities for assigned studies.
Things to Carry for Interview:
- Updated copy of TCS application form with resume
- Original & photocopy of Government ID proof (Aadhar Card/PAN Card/Passport/Voter’s ID) & 1 Passport size photo
- Degree certificate or provisional certificate
- SSC, HSC & all semester’s marksheet
About TCS:
Tata Consultancy Services (TCS) is a global leader in IT services, consulting, and business solutions. With a presence in 46 countries, TCS has been partnering with many of the world’s largest businesses in their transformation journeys for over 50 years. TCS offers a consulting-led, cognitive-powered, integrated portfolio of business, technology, and engineering services and solutions.