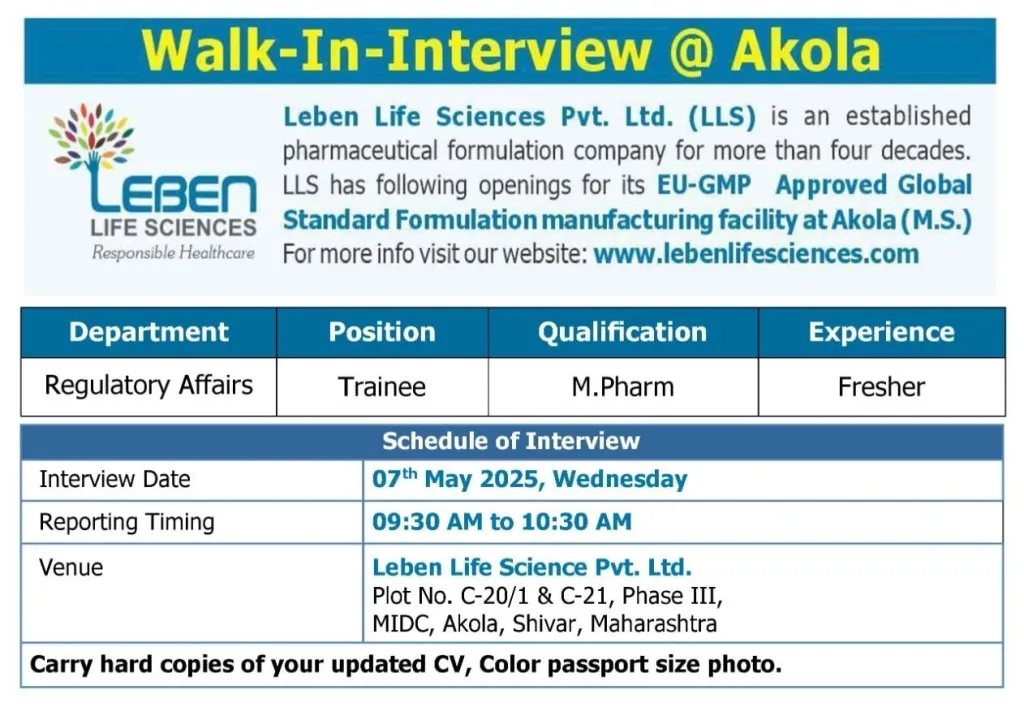
Leben Life Sciences Pvt. Ltd. (LLS), a leading pharmaceutical formulation company with over four decades of experience, is thrilled to announce a fantastic opportunity for fresh graduates seeking a career in the dynamic field of regulatory affairs. We are currently seeking highly motivated and detail-oriented individuals to join our team as Trainees at our EU-GMP Approved Global Standard Formulation manufacturing facility in Akola, Maharashtra.
About Leben Life Sciences Pvt. Ltd.
For over 40 years, Leben Life Sciences has been a trusted name in the pharmaceutical industry, committed to delivering high-quality formulations that meet the strictest global standards. Our EU-GMP approved facility in Akola reflects our unwavering dedication to excellence and compliance. We pride ourselves on fostering a supportive and collaborative work environment where employees can thrive and contribute to our continued success. Learn more about our company and our commitment to innovation at http://www.lebenlifesciences.com.
Trainee Regulatory Affairs – Akola, Maharashtra
This is an exceptional opportunity for recent M.Pharma graduates to launch their careers within a reputable and growing pharmaceutical company. As a Trainee in our Regulatory Affairs department, you will gain invaluable hands-on experience, working alongside experienced professionals and contributing to critical regulatory processes. This role offers extensive learning opportunities and the chance to make a significant impact on our company’s success.
Key Responsibilities (These will be further discussed during the interview process):
- Supporting the preparation and submission of regulatory documents.
- Assisting with the maintenance of regulatory files and records.
- Contributing to the development and implementation of regulatory strategies.
- Conducting research and analysis on relevant regulatory guidelines and requirements.
- Collaborating with cross-functional teams to ensure regulatory compliance.
Ideal Candidate Profile:
- Education: M.Pharma degree from a recognized university.
- Experience: Freshers are strongly encouraged to apply.
- Skills: Strong attention to detail, excellent organizational skills, proficient in Microsoft Office Suite, and a demonstrated ability to work both independently and as part of a team.
- Attributes: Highly motivated, enthusiastic, quick learner, and a strong desire to pursue a career in regulatory affairs.
Walk-in Interview Details:
We invite all interested candidates to attend a walk-in interview at our Akola facility.
- Date: Wednesday, May 7th, 2025
- Time: 9:30 AM to 10:30 AM
- Venue: Leben Life Sciences Pvt. Ltd., Plot No. C-20/1 & C-21, Phase III, MIDC, Akola, Shivar, Maharashtra
Please bring your updated resume and any relevant documents to the interview.
Why Choose Leben Life Sciences?
- Growth Opportunities: We are committed to investing in our employees’ development and providing opportunities for career advancement.
- Collaborative Environment: Work alongside a team of experienced professionals in a supportive and collaborative setting.
- Industry Leader: Be a part of a leading pharmaceutical company with a strong reputation for quality and innovation.
- Global Standards: Gain experience working in a state-of-the-art, EU-GMP approved facility.
How to Apply:
Interested candidates are encouraged to submit their resumes to:
- Email: career@lebenlifesciences.com
- Whatsapp: +91 7498035480
We look forward to receiving your application and welcoming you to the Leben Life Sciences team! This is a fantastic opportunity to start a rewarding career in the pharmaceutical industry. Don’t miss out!