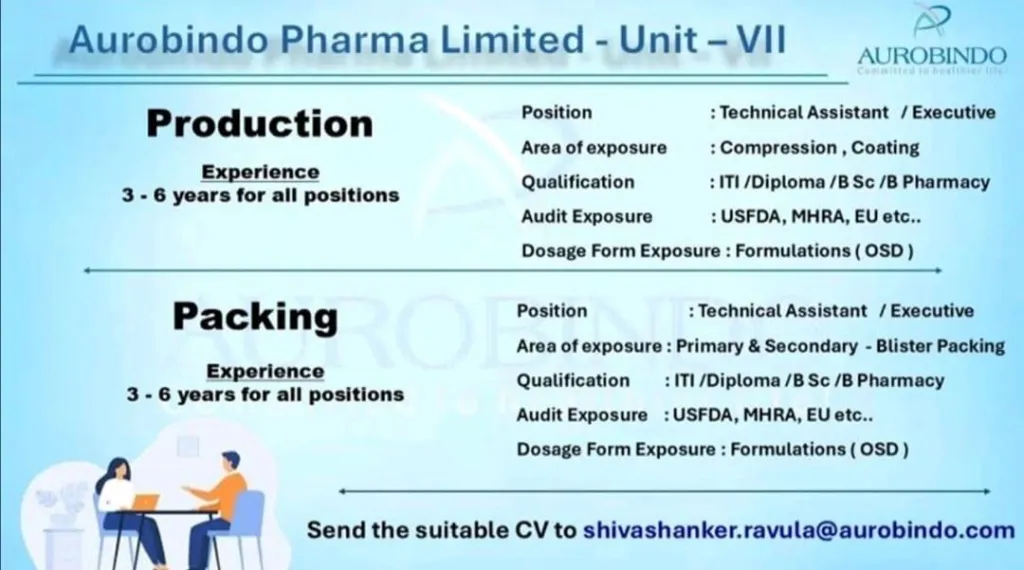
Aurobindo Pharma Limited (APL), a leading global pharmaceutical company, is expanding its OSD (Oral Solid Dosage) Production and Packing Department at its Jadcherla (Unit VII) facility. The company is seeking skilled Technicians and Executives with experience in formulation (OSD) manufacturing and packing operations.
Key Responsibilities:
Production Department:
- Operate and monitor compression and coating machines in OSD production.
- Ensure compliance with USFDA, MHRA, and EU regulatory standards.
- Maintain documentation and follow GMP guidelines.
Packing Department:
- Handle primary and secondary packing operations (blister packing).
- Ensure quality checks and adherence to global audit standards.
About Aurobindo Pharma Ltd (APL)
Aurobindo Pharma is a top-tier Indian multinational pharmaceutical company with:
- Revenue of over $2.8 Billion (2018-19)
- Presence in 34+ countries, exporting to 155 nations
- 2nd Largest Generic Company in the USA (by Rx volume)
- Top 10 Generic Player in Europe (France, Germany, UK, Italy, etc.)
The company is known for its high-quality, affordable generic medicines and globally compliant manufacturing facilities.
Eligibility Criteria
For Technician Roles:
- Education: ITI / Diploma / B.Sc. / B.Pharmacy
- Experience: 3-6 years in OSD production or packing
- Exposure: USFDA/MHRA/EU audits
For Executive Roles:
- Education: B.Pharm / M.Pharm
- Experience: 3-6 years in OSD manufacturing or packing
- Audit Exposure: Regulatory compliance (USFDA, MHRA, EU)
How to Apply
Interested candidates meeting the eligibility criteria can send their updated resume to:
Subject Line: “Application for [Technician/Executive] – OSD Production/Packing – [Your Experience] Years”